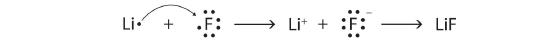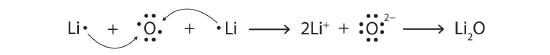4 5 What Is an Ionic Bond Worksheet
3.E: Ionic Bonding and Simple Ionic Compounds (Exercises I)
-
- Last updated
- Save as PDF
- Page ID
- 16172
These are homework exercises to accompany Chapter 3 of the Ball et al. "The Basics of GOB Chemistry" Textmap.
3.1: Two Types of Bonding
Concept Review Exercises
-
What is the octet rule?
-
How are ionic bonds formed?
Answers
-
The octet rule is the concept that atoms tend to have eight electrons in their valence electron shell.
-
Ionic bonds are formed by the attraction between oppositely charged ions.
Exercises
-
Why is an ionic compound unlikely to consist of two positively charged ions?
-
Why is an ionic compound unlikely to consist of two negatively charged ions?
-
A calcium atom has two valence electrons. Do you think it will lose two electrons or gain six electrons to obtain an octet in its outermost electron shell?
-
An aluminum atom has three valence electrons. Do you think it will lose three electrons or gain five electrons to obtain an octet in its outermost electron shell?
-
A selenium atom has six valence electrons. Do you think it will lose six electrons or gain two electrons to obtain an octet in its outermost electron shell?
-
An iodine atom has seven valence electrons. Do you think it will lose seven electrons or gain one electron to obtain an octet in its outermost electron shell?
Answers
-
Positive charges repel each other, so an ionic compound is not likely between two positively charged ions.
3. It is more likely to lose two electrons.
5. It is more likely to gain two electrons.
3.2: Ions
Concept Review Exercises
-
What are the two types of ions?
-
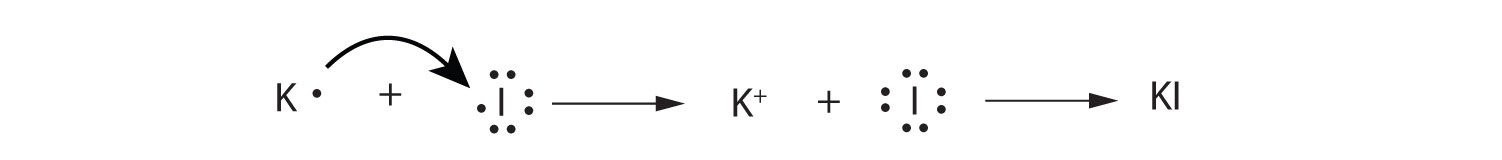
Use Lewis diagrams to illustrate the formation of an ionic compound from a potassium atom and an iodine atom.
-
When the following atoms become ions, what charges do they acquire?
- Li
- S
- Ca
- F
Answers
-
Cations have positive charges, and anions have negative charges.
-
-
- 1+
- 2−
- 2+
- 1−
Key Takeaways
- Ions can be positively charged or negatively charged.
- A Lewis diagram is used to show how electrons are transferred to make ions and ionic compounds.
Exercises
-
Identify each as a cation, an anion, or neither.
- H+
- Cl−
- O2
- Ba2 +
- CH4
- CS2
-
Identify each as a cation, an anion, or neither.
- NH3
- Br−
- H−
- Hg2 +
- CCl4
- SO3
-
Write the electron configuration for each ion.
- Li+
- Mg2 +
- F−
- S2−
-
Write the electron configuration for each ion.
- Na+
- Be2 +
- Cl−
- O2−
-
Draw Lewis diagrams for the ions listed in Exercise 3. Also include Lewis diagrams for the respective neutral atoms as a comparison.
-
Draw Lewis diagrams for the ions listed in Exercise 4. Also include Lewis diagrams for the respective neutral atoms as a comparison.
-
Using Lewis diagrams, show the electron transfer for the formation of LiF.
-
Using Lewis diagrams, show the electron transfer for the formation of MgO.
-
Using Lewis diagrams, show the electron transfer for the formation of Li2O.
-
Using Lewis diagrams, show the electron transfer for the formation of CaF2.
-
What characteristic charge do atoms in the first column of the periodic table have when they become ions?
-
What characteristic charge do atoms in the second column of the periodic table have when they become ions?
-
What characteristic charge do atoms in the third-to-last column of the periodic table have when they become ions?
-
What characteristic charge do atoms in the next-to-last column of the periodic table have when they become ions?
Answers
1.
- cation
- anion
- neither
- cation
- neither
- neither
3.
- 1s 2
- 1s 22s 22p 6
- 1s 22s 22p 6
- 1s 22s 22p 63s 23p 6
5.
-
1+
3.3: Formulas for Ionic Compounds
Concept Review Exercises
-
What information is contained in the formula of an ionic compound?
-
Why do the chemical formulas for some ionic compounds contain subscripts, while others do not?
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- Mg2 + and I−
- Na+ and O2−
Answers
-
the ratio of each kind of ion in the compound
-
Sometimes more than one ion is needed to balance the charge on the other ion in an ionic compound.
-
- MgI2
- Na2O
Key Takeaways
- Proper chemical formulas for ionic compounds balance the total positive charge with the total negative charge.
- Groups of atoms with an overall charge, called polyatomic ions, also exist.
Exercises
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- Na+ and Br−
- Mg2 + and Br−
- Mg2 + and S2−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- K+ and Cl−
- Mg2 + and Cl−
- Mg2 + and Se2−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- Na+ and N3−
- Mg2 + and N3−
- Al3 + and S2−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- Li+ and N3−
- Mg2 + and P3−
- Li+ and P3−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- Fe3 + and Br−
- Fe2 + and Br−
- Au3 + and S2−
- Au+ and S2−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- Cr3 + and O2−
- Cr2 + and O2−
- Pb2 + and Cl−
- Pb4 + and Cl−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- Cr3 + and NO3 −
- Fe2 + and PO4 3−
- Ca2 + and CrO4 2−
- Al3 + and OH−
-
Write the chemical formula for the ionic compound formed by each pair of ions.
- NH4 + and NO3 −
- H+ and Cr2O7 2−
- Cu+ and CO3 2−
- Na+ and HCO3 −
-
For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them.
- Ba and S
- Cs and I
-
For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them.
- K and S
- Sc and Br
-
Which compounds would you predict to be ionic?
- Li2O
- (NH4)2O
- CO2
- FeSO3
- C6H6
- C2H6O
-
Which compounds would you predict to be ionic?
- Ba(OH)2
- CH2O
- NH2CONH2
- (NH4)2CrO4
- C8H18
- NH3
Answers
-
- NaBr
- MgBr2
- MgS
-
- Na3N
- Mg3N2
- Al2S3
-
- FeBr3
- FeBr2
- Au2S3
- Au2S
-
- Cr(NO3)3
- Fe3 (PO4)2
- CaCrO4
- Al(OH)3
-
- Ba2 +, S2−, BaS
- Cs+, I−, CsI
-
- ionic
- ionic
- not ionic
- ionic
- not ionic
- not ionic
3.4: Ionic Nomenclature
Concept Review Exercises
-
Briefly describe the process for naming an ionic compound.
-
In what order do the names of ions appear in the names of ionic compounds?
-
Which ionic compounds can be named using two different systems? Give an example.
Answers
-
Name the cation and then the anion but don't use numerical prefixes.
-
the cation name followed by the anion name
-
Ionic compounds in which the cation can have more than one possible charge have two naming systems. FeCl3 is either iron(III) chloride or ferric chloride (answers will vary).
Key Takeaways
- Each ionic compound has its own unique name that comes from the names of the ions.
Exercises
-
Name each ion.
- Ra2 +
- P3−
- H2PO4 −
- Sn4 +
-
Name each ion.
- Cs+
- As3−
- HSO4 −
- Sn2 +
-
Name the ionic compound formed by each pair of ions.
- Na+ and Br−
- Mg2 + and Br−
- Mg2 + and S2−
-
Name the ionic compound formed by each pair of ions.
- K+ and Cl−
- Mg2 + and Cl−
- Mg2 + and Se2−
-
Name the ionic compound formed by each pair of ions.
- Na+ and N3−
- Mg2 + and N3−
- Al3 + and S2−
-
Name the ionic compound formed by each pair of ions.
- Li+ and N3−
- Mg2 + and P3−
- Li+ and P3−
-
Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Fe3 + and Br−
- Fe2 + and Br−
- Au3 + and S2−
- Au+ and S2−
-
Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Cr3 + and O2−
- Cr2 + and O2−
- Pb2 + and Cl−
- Pb4 + and Cl−
-
Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- Cr3 + and NO3 −
- Fe2 + and PO4 3−
- Ca2 + and CrO4 2−
- Al3 + and OH−
-
Name the ionic compound formed by each pair of ions. Use both the Stock and common systems, where appropriate.
- NH4 + and NO3 −
- H+ and Cr2O7 2−
- Cu+ and CO3 2−
- Na+ and HCO3 −
-
Give two names for each compound.
- Al(HSO4)3
- Mg(HSO4)2
-
Give two names for each compound.
- Co(HCO3)2
- LiHCO3
Answers
-
- the radium ion
- the phosphide ion
- the dihydrogen phosphate ion
- the tin(IV) ion or the stannic ion
-
- sodium bromide
- magnesium bromide
- magnesium sulfide
-
- sodium nitride
- magnesium nitride
- aluminum sulfide
-
- iron(III) bromide or ferric bromide
- iron(II) bromide or ferrous bromide
- gold(III) sulfide or auric sulfide
- gold(I) sulfide or aurous sulfide
-
- chromium(III) nitrate or chromic nitrate
- iron(II) phosphate or ferrous phosphate
- calcium chromate
- aluminum hydroxide
-
- aluminum hydrogen sulfate or aluminum bisulfate
- magnesium hydrogen sulfate or magnesium bisulfate
3.5: Formula Mass
Concept Review Exercises
-
What is the relationship between atomic mass and formula mass?
-
How are subscripts used to determine a formula mass when more than one polyatomic ion is present in a chemical formula?
Answers
-
The formula mass is the sum of the atomic masses of the atoms in the formula.
-
The subscript is distributed throughout the parentheses to determine the total number of atoms in the formula.
Key Takeaways
- Formula masses of ionic compounds can be determined from the masses of the atoms in their formulas.
Exercises
-
What is the formula mass for the ionic compound formed by each pair of ions?
- Na+ and Br−
- Mg2 + and Br−
- Mg2 + and S2−
-
What is the formula mass for the ionic compound formed by each pair of ions?
- K+ and Cl−
- Mg2 + and Cl−
- Mg2 + and Se2−
-
What is the formula mass for the ionic compound formed by each pair of ions?
- Na+ and N3−
- Mg2 + and N3−
- Al3 + and S2−
-
What is the formula mass for the ionic compound formed by each pair of ions?
- Li+ and N3−
- Mg2 + and P3−
- Li+ and P3−
-
What is the formula mass for each compound?
- FeBr3
- FeBr2
- Au2S3
- Au2S
-
What is the formula mass for each compound?
- Cr2O3
- CrO
- PbCl2
- PbCl4
-
What is the formula mass for each compound?
- Cr(NO3)3
- Fe3(PO4)2
- CaCrO4
- Al(OH)3
-
What is the formula mass for each compound?
- NH4NO3
- H2Cr2O7
- Cu2CO3
- NaHCO3
-
What is the formula mass for each compound?
- Al(HSO4)3
- Mg(HSO4)2
-
What is the formula mass for each compound?
- Co(HCO3)2
- LiHCO3
Answers
-
- 102.90 amu
- 184.11 amu
- 56.38 amu
-
- 83.00 amu
- 100.93 amu
- 150.17 amu
-
- 295.50 amu
- 215.60 amu
- 490.30 amu
- 426.10 amu
-
- 238.00 amu
- 357.49 amu
- 156.08 amu
- 78.01 amu
-
- 318.22 amu
- 218.47 amu
3.6: Chapter Summary
Q3.1.1
Why is an ionic compound unlikely to consist of two positively charged ions?
A3.1.1
Positive charges repel each other, so an ionic compound is not likely between two positively charged ions.
H3.1.1
Think of Coulombs law.
S3.1.1
Coulomb's law relates the force of interaction between two charged species.
\[F=\dfrac{Q_1Q_2}{4\pi\epsilon R_{12}}\]
Hence there is a repulsion (\(-F\)) between species of same polarity.
Q3.1.2
Why is an ionic compound unlikely to consist of two negatively charged ions?
Q3.1.3
A calcium atom has two valence electrons. Do you think it will lose two electrons or gain six electrons to obtain an octet in its outermost electron shell?
A.3.1.3: It is more likely to lose two electrons.
Q3.1.4
An aluminum atom has three valence electrons. Do you think it will lose three electrons or gain five electrons to obtain an octet in its outermost electron shell?
Q3.1.5
A selenium atom has six valence electrons. Do you think it will lose six electrons or gain two electrons to obtain an octet in its outermost electron shell?
A3.1.5: It is more likely to gain two electrons.
Q3.1.6
An iodine atom has seven valence electrons. Do you think it will lose seven electrons or gain one electron to obtain an octet in its outermost electron shell?
4 5 What Is an Ionic Bond Worksheet
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Exercises%3A_General_Organic_and_Biological_Chemistry/Exercises%3A_General_Organic_and_Biological_Chemistry_(Ball_et_al.)/03.E%3A_Exercises